The structural reconstruction at the crystal layer edges of 2D lead halide perovskites (LHPs) leads to unique edge states (ES), which are manifested by prolonged carrier lifetime and reduced emission energy. These special ES can effectively enhance the optoelectronic performance of devices, but their intrinsic origin and working mechanism remain elusive. Here it is demonstrated that the ES of a family of 2D Ruddlesden–Popper LHPs [BA2CsPb2Br7, BA2MAPb2Br7, and BA2MA2Pb3Br10 (BA = butylammonium; MA = methylammonium)] arise from the rotational symmetry elevation of the PbBr6 octahedra dangling at the crystal layer edges. These dangling octahedra give rise to localized electronic states that enable an effective transport of electrons from the interior to layer edges, and the population of electrons in both the interior states and the ES can be manipulated via controlling the external fields. Moreover, the abundant phonons, activated by the dangling octahedra, can interact with electrons to facilitate radiative recombination, counterintuitive to the suppressive role commonly observed in conventional semiconductors. This work unveils the intrinsic atomistic and electronic origins of ES in 2D LHPs, which can stimulate the exploration of ES-based exotic optoelectronic properties and the corresponding design of high-performance devices for these emergent low-dimensional semiconductors.
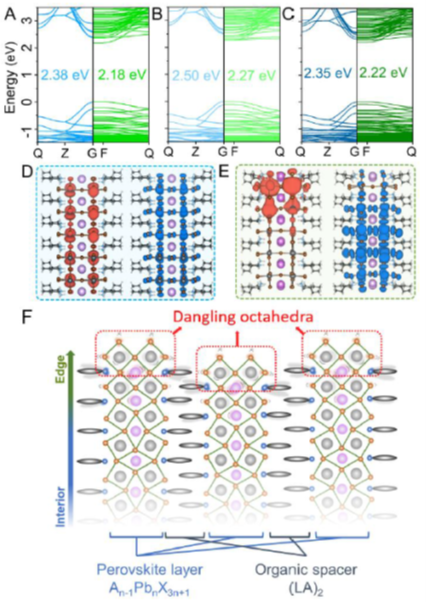
The electronic origin of ES enabled by dangling octahedra at the layer edge in 2D LHPs. (A-C) DFT calculated band structures and band gap values of BA2CsPb2Br7 (A), BA2MAPb2Br7 (B) and BA2MA2Pb3Br10 (C); the results of Bulk mode and Edge containing model are plotted as blue and green lines, respectively. (D-E) The partial charge densities of the conduction band minima (left panel) and the valence band maxima (right panel) derived from the Bulk model (D) and Edge-containing model (E) of BA2CsPb2Br7. (F) Scheme of proposed dangling octahedra which lead to ES in RP perovskites, (LA)2An-1PbnX3n+1, where LA is large amine (BA or PEA); A is monovalent cation (MA, FA or Cs); X is Br or I.
Article published in: Advanced Materials